Eco-friendly Suzuki–Miyaura coupling of arylboronic acids to aromatic ketones catalyzed by the oxime-palladacycle in biosolvent 2-MeTHF†
Abstract
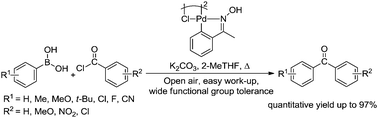
Oxime-palladacycle catalyzed Suzuki–Miyaura cross-coupling reaction of acid chlorides and arylboronic acids to yield aryl ketones was developed. The remarkable benefit of this method is the use of water immiscible (practically) 2-MeTHF as solvent, which provides easy isolation of the crude reaction mixture just by separation of 2-MeTHF–water layers, and then evaporation of 2-MeTHF. Moreover, the use of relatively equal proportion of substrates and less generation of side products make the method highly atom economic.

 Please wait while we load your content...
Please wait while we load your content...