Catalytic n-pentane conversion on H-ZSM-5 at high pressure†
Abstract
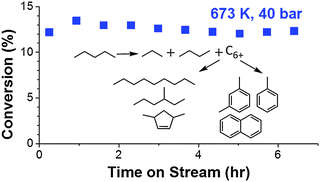
The effect of temperature (633–723 K), pressure (10–60 bar) and weight hourly space velocity (WHSV) (400–1500 gC5 gcat−1 h−1) on the conversion of n-pentane on H-[Al]ZSM-5 type catalysts has been investigated. Catalyst properties were tested using a packed-bed laboratory microreactor and reaction products were analyzed via online gas chromatography. 5–25% pentane conversion was observed at a pressure of 40 bar and temperatures in the range of 633–723 K. Reactant consumption rate approached saturation kinetics at pressures above 30 bar (∼14% conversion, 673 K). At 40 bar and 673 K, increasing WHSV (400–1500 gC5 gcat−1 h−1) resulted in a reduction in pentane conversion (26–10%). In all cases, propane and butane were the major products, followed by heavier C6+ compounds and other lighter products (C1–C4 paraffins and olefins). Propane carbon selectivity increased from 24% at 633 K to 34% at 723 K, while butane carbon selectivity (∼40%) was nearly constant. An inverse relationship between the production of C6+ and light products was observed with changes in reaction conditions. The carbon selectivity to C6+ compounds increased from 20% at 10 bar to 27% at 60 bar and decreased from 28% at 633 K to 18% at 723 K. At all reaction conditions, the observed product distribution can be explained as the result of fast bimolecular reactions, including hydride transfer and alkylation.
- This article is part of the themed collection: The Creative World of Porous Materials

 Please wait while we load your content...
Please wait while we load your content...