Two-step synthesis of nanocomposite LiFePO4/C cathode materials for lithium ion batteries
Abstract
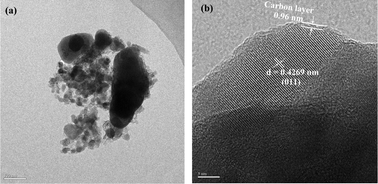
A two-step method is developed for the preparation of nanocomposite LiFePO4/C cathode materials for lithium ion batteries. Water is used as a solvent in the sintering process, with Fe(CH3COO)2·4H2O and H3PO4 as the raw materials and citric acid as the carbon source. The synthesis of the resultant grain-size precursor is then performed at 700 °C for 1 h in order to obtain a lithiation reaction precursor via ball milling. The crystal structure and morphology of the samples are characterized using X-ray diffraction, scanning electron microscopy and transmission electron microscopy. The electrochemical properties of the material are assessed using charge–discharge and cyclic voltammetry testing. Based on the test results, the discharge capacity of LiFePO4/C reaches 163.3 mA h g−1 in the first cycle, which is close to the theoretical value (170 mA h g−1). After 50 charge–discharge cycles, a capacity of 154.4 mA h g−1 is obtained. The capacity retention ratio is 94.5%.

 Please wait while we load your content...
Please wait while we load your content...