Hierarchically porous Li1.2Mn0.6Ni0.2O2 as a high capacity and high rate capability positive electrode material
Abstract
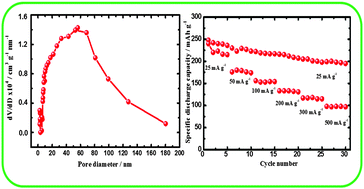
Layered composite samples of lithium-rich manganese oxide (Li1.2Mn0.6Ni0.2O2) are prepared by a reverse microemulsion route employing a soft polymer template and studied as a positive electrode material. The product samples possess dual porosity with distribution of pores at 3.5 and 60 nm. Pore volume and surface area decrease on increasing the temperature of preparation. Nevertheless, the electrochemical activity of the composite increases with an increase in temperature. The discharge capacity value of the samples prepared at 800 and 900 °C is about 240 mA h g−1 at a specific current of 25 mA g−1 with a good cycling stability. The composite sample heated at 900 °C possesses a high rate capability with a discharge capacity of 100 mA h g−1 at a specific current of 500 mA g−1. The high rate capability is attributed to porous nature of the composite sample.

 Please wait while we load your content...
Please wait while we load your content...