Influences of the protonic state of an imidazole-phenanthroline ligand on the luminescence properties of copper(i) complexes: experimental and theoretical research†
Abstract
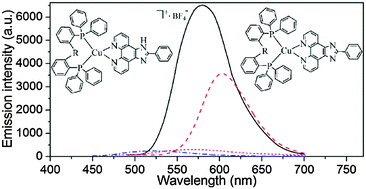
Two ionic (1a and 1b) and two neutral (2a and 2b) Cu(I) complexes containing an un-deprotonated or a deprotonated nitrogen ligand {2-(4-methyl phenyl) imidazole[4,5-f]-1,10-phenanthroline, MHPIP} and different phosphine ligands (bis[2-(diphenylphosphino) phenyl]ether and PPh3) have been synthesized and characterized by elemental analysis, 1H NMR spectroscopy and X-ray crystallography (1b, 2a and 2b). The complexes adopt a distorted tetrahedral geometry constructed by MHPIP (or MPIP−) and phosphine ligands. The emission spectra show that the ionic complexes exhibit almost ignorable luminescence. However, the deprotonation of the nitrogen ligand makes the neutral complexes exhibit orange or yellow emission both in solution and solid-powder states. Considering the different luminous characters of the neutral complexes, density functional theory (DFT) calculations have been performed at the B3LYP/6-31G** level to provide information about the impact of phosphine ligands on the frontier orbital.

 Please wait while we load your content...
Please wait while we load your content...