Destabilization of the metal site as a hub for the pathogenic mechanism of five ALS-linked mutants of copper, zinc superoxide dismutase†
Abstract
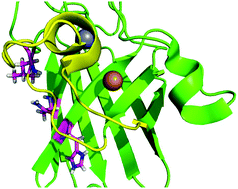
Amyotrophic lateral sclerosis (ALS) is a lethal neurodegenerative disease, with no effective pharmacological treatment. Its pathogenesis is unknown, although a subset of the cases is linked to genetic mutations. A significant fraction of the mutations occur in one protein, copper, zinc superoxide dismutase (SOD1). The toxic function of mutant SOD1 has not been elucidated, but damage to the metal site of the protein is believed to play a major role. In this work, we study the electrostatic loop of SOD1, which we had previously proposed to work as a “solvent seal” isolating the metal site from water molecules. Out of the five contact points identified between the electrostatic loop and its dock in the rest of the protein, three points were found to be affected by ALS-linked mutations, with a total of five mutations identified. The effect of the five mutations was studied using methods of computational chemistry. We found that four of the mutations destabilize the proposed solvent seal, while the fifth mutation directly affects the metal-site stability. In the two contact points unaffected by ALS-linked mutations, the side chains of the residues were not found to play a stabilizing role. Our results show that the docking of the electrostatic loop to the rest of SOD1 plays a role in ALS pathogenesis, in support of that structure acting as a solvent barrier for the metal site. The results provide a unified pathogenic mechanism for five different ALS-linked mutations of SOD1.
- This article is part of the themed collection: Zinc in the Biosciences
 Please wait while we load your content...
Please wait while we load your content...