Comparative metalloproteomic approaches for the investigation proteins involved in the toxicity of inorganic and organic forms of mercury in rice (Oryza sativa L.) roots
Abstract
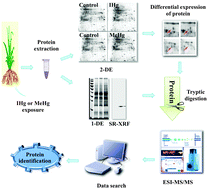
The toxicity mechanisms of rice roots under inorganic mercury (IHg) or methylmercury (MeHg) stress were investigated using metalloproteomic approaches. Rice seedlings were cultivated in nutrient solutions with IHg or MeHg for three weeks. Proteins were extracted from the roots and separated by two-dimensional electrophoresis (2-DE). Differentially expressed proteins were analyzed using ESI-MS/MS and identified by PMF. 26 and 29 protein spots were differentially expressed in the IHg- and MeHg-exposed roots, respectively. The proteins responsive to Hg exposure are involved in antioxidative defense, sulfur and glutathione metabolism, carbohydrate and energy metabolism, programmed cell death, and pathogen defense. Chitinase and salt stress-induced proteins exhibited a greater differentially expression in response to MeHg stress compared to IHg stress. Hg-binding proteins were detected by the combined use of 1-DE, SRXRF, and ESI-MS/MS. The results showed that Hg was bound to proteins of 15–25 kDa in rice roots under Hg stress. The Hg contents in the band under IHg stress were remarkably higher than those under MeHg. Hg binds to proteins, which leads to irreversible damage of root growth. Rice roots changed the related protein expression levels in response to Hg stress. These results may provide new insights into the mechanism of toxicity of IHg and MeHg in rice.
- This article is part of the themed collection: Fifth International Symposium on Metallomics, Beijing, China

 Please wait while we load your content...
Please wait while we load your content...