Abstract
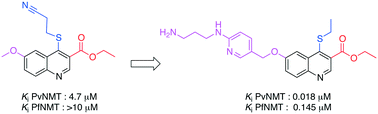
The parasite Plasmodium vivax is the most widely distributed cause of recurring malaria. N-Myristoyltransferase (NMT), an enzyme that catalyses the covalent attachment of myristate to the N-terminal glycine of substrate proteins, has been described as a potential target for the treatment of this disease. Herein, we report the synthesis and the structure-guided optimization of a series of quinolines with balanced activity against both Plasmodium vivax and Plasmodium falciparum N-myristoyltransferase (NMT).

 Please wait while we load your content...
Please wait while we load your content...