Novel tetrazoloquinoline–thiazolidinone conjugates as possible antitubercular agents: synthesis and molecular docking†‡
Abstract
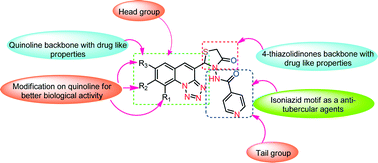
A novel approach for the synthesis of a new 4-thiazolidinone scaffold was developed by a one-pot three-component cyclocondensation of various tetrazolo quinoline aldehydes 1a–f, acid hydrazide 2a–c, and thioglycolic acid 3 in the presence of [DBUH][OAc] as a catalyst in high yields. All the conjugates were screened for their antimycobacterial activity against MTB H37Ra and M. bovis BCG strains, with the MIC values ranging from 0.99–13.55 μmol mL−1 and 0.14–20.11 μmol mL−1, respectively. The 4-thiazolidinone-incorporated tetrazoloquinoline derivatives 4a, 4d, 4g, 4j, 4m, and 4p were highly potent against MTB H37Ra and M. bovis BCG strains. The most active compounds were also evaluated for their cytotoxicity against MCF-7, A549, and HCT 116 cell lines and were found to be non-cytotoxic. Further, molecular docking studies into the active site of the InhA enzyme revealed a similar binding mode to the native ligand in the crystal structure, thereby helping us to understand the ligand–protein binding interaction and establish a structural basis for the inhibition of mycobacterium tuberculosis. The results suggest that the tetrazoloquinoline–thiazolidinone conjugates 4a, 4d, 4g, 4j, 4m, and 4p are promising antitubercular agents.

 Please wait while we load your content...
Please wait while we load your content...