Abstract
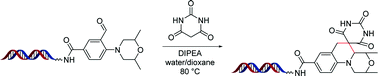
The generation of novel chemical leads for clinical development is a constant challenge in the pharmaceutical industry. The synthesis of DNA-encoded libraries has emerged as a powerful method for hit and lead generation. We report the development of the tertiary amino effect reaction on DNA-tethered substrates. A variety of ortho-dialkylaminoaryl aldehydes undergo a cascade reaction involving a Knoevenagel condensation, a [1,5]-hydride shift, and a Mannich cyclization to give diversely substituted spirocycles. NMR analysis of substrates bearing an enriched double-13C label confirmed product formation. The net formation of two carbon–carbon bonds adds to the few examples of carbon–carbon forming reactions performed in presence of DNA-encoding systems.

 Please wait while we load your content...
Please wait while we load your content...