Abstract
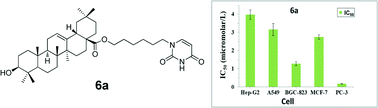
A total of 16 targeted oleanolic acid–uracil/thymine hybrids were designed and synthesized as potential cytotoxic agents. Most IC50 values were under 10.0 μM, with some of them under 0.1 μM in vitro test against tested cells (Hep-G2, A549, BGC-823, MCF-7 and PC-3). These hybrids displayed much more potent inhibitory activities compared with oleanolic acid and commercial anticancer drug 5-fluorouracil (5-FU). The combination of fluorescence staining observation and flow cytometric analysis suggested that selected oleanolic acid–uracil hybrid 6a possessed clear cell apoptosis inducing effects. And the underlying mechanisms for the antitumor activity were associated with loss of the mitochondrial membrane potential, arrest of the Hep-G2 cell line at the G1 phase and activation of effector caspase-3/9 to trigger cell apoptosis.

 Please wait while we load your content...
Please wait while we load your content...