Biological evaluation of quinoline derivatives as inhibitors of human dihydroorotate dehydrogenase†
Abstract
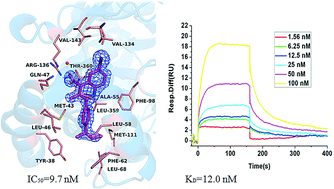
Human dihydroorotate dehydrogenase (hDHODH) is an enzyme that catalyzes the fourth step in de novo pyrimidine biosynthesis, and its inhibitors restrict the growth of rapidly proliferating cells. Therefore, hDHODH has been reported as an attractive target for the treatment of cancer and autoimmune diseases. In this study, several quinoline derivatives were identified as potent inhibitors against hDHODH, among which compound A9 was the most potent one with an IC50 value of 9.7 nM. We further verified by thermal shift assay (TSA), surface plasmon resonance (SPR) and X-ray crystallography that A9 could directly bind to the target hDHODH. The crystal structure of hDHODH in complex with compound A9 was refined to 1.90 Å and the binding mode of compound A9 was summarized. Moreover, structure–activity relationship (SAR) analysis of quinoline derivatives with hDHODH indicated that quinoline derivatives with a carboxyl group in R1, a bromine atom in R2 and a para-alkyl-substituted phenyl group in R5, were beneficial for potency against hDHODH, which plays an important role in inhibitor design and optimization.

 Please wait while we load your content...
Please wait while we load your content...