Antiprotozoal activity of dehydroabietic acid derivatives against Leishmania donovani and Trypanosoma cruzi†
Abstract
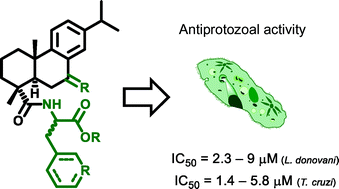
Derivatives of dehydroabietic acid bearing different amino acids scaffolds have potent antiprotozoal activity against Leishmania donovani and Trypanosoma cruzi, with good to high selectivity, and can therefore be regarded as good models for further development into new drugs to fight leishmaniasis and Chagas disease. Several of the tested compounds were able to kill parasites residing inside cells, with IC50 values ranging from 2.3 to 9 μM (L. donovani) and 1.4 to 5.8 μM (T. cruzi), reflecting their ability to fight these infections at the relevant stage responsible for disease. One of the compounds, bearing a 3-pyridyl-D-alanine side chain, was 1.5-fold more potent against T. cruzi amastigotes residing in L6 cells than the reference compound benznidazole.


 Please wait while we load your content...
Please wait while we load your content...