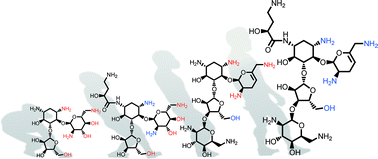
Structural hybridization of three aminoglycoside antibiotics yields a potent broad-spectrum bactericide that eludes bacterial resistance enzymes†
Abstract
Vast numbers of prevalent aminoglycoside-modifying enzymes undermine the clinical use of aminoglycoside antibiotics. We present the design and synthesis of a potent broad-spectrum bactericidal aminoglycoside based on available X-ray co-crystal structures within the ribosomal binding-site. The resulting antibiotic displays broad protection of its functional groups from inactivation by clinically relevant resistance enzymes.

 Please wait while we load your content...
Please wait while we load your content...