Tricyclic phenothiazine and phenoselenazine derivatives as potential multi-targeting agents to treat Alzheimer's disease
Abstract
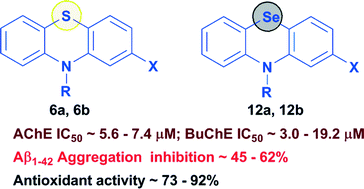
A group of tricyclic phenothiazines (6a, 6b and 7a–l) and phenoselenazines (12a, 12b and 13a–l) was designed, synthesized and evaluated as multi-targeting ligands aimed at the cholinergic, amyloid and oxidative stress pathways of Alzheimer's disease. The phenothiazine derivative 7j (2-chloro-10H-phenothiazin-10-yl-(4-methoxyphenyl)methanone) was identified as the best dual, non-selective cholinesterase inhibitor (AChE IC50 = 5.9 ± 0.6 μM; BuChE IC50 = 5.3 ± 0.5 μM), whereas in the corresponding phenoselenazine series, 13j (2-chloro-10H-phenoselenazin-10-yl-(4-methoxyphenyl)methanone) exhibited good non-selective cholinesterase inhibition (AChE IC50 = 5.8 ± 0.4 μM; BuChE IC50 = 4.9 ± 0.5 μM). Interestingly, N-10 unsubstituted phenothiazine 6a (AChE IC50 = 7.3 ± 0.6 μM; BuChE IC50 = 5.8 ± 0.5 μM; Aβ1–42 aggregation inhibition = 62%; DPPH scavenging = 92%), and the corresponding phenoselenazine bioisostere 12a (AChE IC50 = 5.6 ± 0.4 μM; BuChE IC50 = 3.0 ± 0.5 μM; Aβ1–42 aggregation inhibition = 45.6%; DPPH scavenging = 84.4%) were able to exhibit multi-targeting ability by demonstrating cholinesterase inhibition, beta-amyloid aggregation and antioxidant properties. These results show that fused tricyclic ring systems based on either phenothiazine or phenoselenazine templates can be useful to develop hybrid small molecules to target multiple pathological routes associated with Alzheimer's disease.

 Please wait while we load your content...
Please wait while we load your content...