In vivo synthesized 34S enriched amino acid standards for species specific isotope dilution of proteins
Abstract
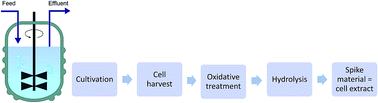
A generic quantification approach was introduced addressing the characterization of protein standards while fulfilling the principles of metrology. Traceable absolute quantification was achieved combining a proven biochemical method, i.e. protein hydrolysis followed by amino acid quantification with the concept of species specific isotope dilution analysis (IDA). The method relies on the determination of the two sulfur containing amino acids, cysteine and methionine by sulfur speciation analysis and is hence applicable to any protein containing sulfur. In vivo synthesis using 34S as sulfur source in yeast fermentations provided species specific isotopically enriched standards for IDA quantification of cysteine and methionine in the oxidized forms, methionine sulfone and cysteic acid. Reverse isotope dilution mass spectrometry (IDMS) characterization by inductively coupled plasma mass spectrometry (ICP-MS) combined to anion exchange showed that very high concentrated spike material could be produced with μmol amounts of proteinogenic sulfur containing amino acids per g cell dry weight. An enrichment of 34S to 96.3 ± 0.4% (n = 3) and 98.5 ± 0.4% (n = 3) for cysteic acid and methionine sulfone, respectively, was assessed. The established IDA method was validated for the absolute quantification of commercially available lysozyme and ceruloplasmin standards including the calculation of a total combined uncertainty budget.
- This article is part of the themed collection: Speciation Analysis

 Please wait while we load your content...
Please wait while we load your content...