Matrix stiffness exerts biphasic control over monocyte–endothelial adhesion via Rho-mediated ICAM-1 clustering†
Abstract
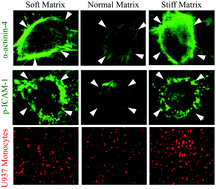
Leukocyte–endothelial adhesion is a critical early step in chronic vascular inflammation associated with diabetes, emphysema, and aging. Importantly, these conditions are also marked by abnormal subendothelial matrix crosslinking (stiffness). Yet, whether and how abnormal matrix stiffness contributes to leukocyte–endothelial adhesion remains poorly understood. Using a co-culture of human monocytic cells and human microvascular endothelial cells (ECs) grown on matrices of tunable stiffness, we demonstrate that matrix stiffness exerts biphasic control over monocyte–EC adhesion, with both matrix softening and stiffening eliciting a two-fold increase in this adhesive interaction. This preferential endothelial adhesivity on softer and stiffer matrices was consistent with a significant increase in α-actinin-4-associated endothelial ICAM-1 clustering, a key determinant of monocyte–EC adhesion. Further, the enhanced ICAM-1 clustering on soft and stiff matrices correlated strongly with an increase in Rho activity and ROCK2 expression. Importantly, inhibition of Rho/ROCK activity blocked the effects of abnormal matrix stiffness on ICAM-1 clustering and monocyte–EC adhesion. Thus, these findings implicate matrix stiffness-dependent ICAM-1 clustering as an important regulator of vascular inflammation and provide the rationale for closely examining mechanotransduction pathways as new molecular targets for anti-inflammatory therapy.

 Please wait while we load your content...
Please wait while we load your content...