Reformatsky and Blaise reactions in flow as a tool for drug discovery. One pot diversity oriented synthesis of valuable intermediates and heterocycles†
Abstract
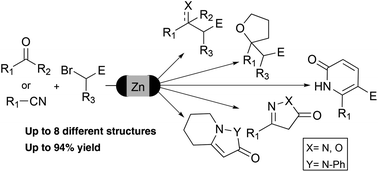
The application of Reformatsky and Blaise reactions for the preparation of a diverse set of valuable intermediates and heterocycles in a one-pot protocol is described. To achieve this goal, a greener activation protocol for zinc in flow conditions has been developed to introduce this metal efficiently into α-bromoacetates. The organozinc compounds were added to a diverse set of ketones and nitriles to obtain a wide range of functional groups and heterocyclic systems.
- This article is part of the themed collection: Continuous processing and flow chemistry in the pharmaceutical industry


 Please wait while we load your content...
Please wait while we load your content...