Fueling biomass-degrading oxidative enzymes by light-driven water oxidation†
Abstract
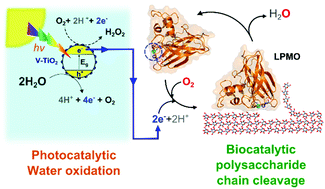
Photosynthesis may be described as light-driven oxidation of water and subsequent use of the generated reducing equivalents to fix CO2 and synthesize higher energy organic compounds, such as carbohydrates. The transposition of the sustainable and atom-efficient strategy of water oxidation to in vitro controlled biocatalytic reactions is poorly studied but is of high interest for the development of photobiocatalysis, and eco-friendly catalytic tools in a wider sense. Here we demonstrate that light-driven oxidation of water catalysed by vanadium-doped TiO2 (V-TiO2), a re-usable photocatalyst, can provide the electrons that lytic polysaccharide monooxygenases (LPMOs) need to oxidatively deconstruct biomass polysaccharides. The demonstration that electrons may be generated by water oxidation alleviates the need for an externally added electron donor, which so far has been a prerequisite for LPMO activity. Importantly, photocatalytic LPMO activation was achieved in the absence of redox mediators, which represents the first demonstration of mediator-free electron transfer from V-TiO2 particles to a redox enzyme, expanding the repertoire of known and conceivable photobiocatalytic reactions. Fundamentally, this photobiocatalytic system allows activation and tight control of LPMO activity, thus offering new tools for mechanistic studies of these industrially important and ubiquitous enzymes. The latter is illustrated by real-time studies of the redox state of an LPMO, using the controllable light-V-TiO2 technology for LPMO reduction and a novel fluorescence method for monitoring re-oxidation. We also show that the light-V-TiO2 technology may be used to study pre-activation of LPMOs.

 Please wait while we load your content...
Please wait while we load your content...