Electrochemical reductive amination of furfural-based biomass intermediates†
Abstract
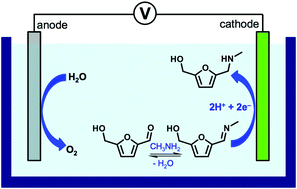
Electrochemical reductive amination uses water as the hydrogen source without requiring other chemicals as the reducing agent, providing reaction conditions that are more environmentally benign. The catalytic abilities and efficiencies of various metal electrodes (Ag, Cu, Pt, Sn, Zn) were investigated for the electrochemical reductive amination of 5-hydroxymethylfurfural (HMF), a key intermediate for biomass conversion, with methylamine (CH3NH2). For each metal electrode, the potentials necessary to initiate reductive amination as well as potential dependent Faradaic efficiency (FE) and selectivity were investigated systematically. Based on the results, reduction conditions and a high surface area Ag electrode that can achieve a FE and a selectivity nearing 100% were reported. Furthermore, reductive amination of HMF derivatives such as 5-methylfurfural (5-MF), 2,5-diformylfuran (DFF), and 5-formyl-2-furancarboxylic acid (FFCA) using methylamine as well as reductive amination of HMF using ethanolamine (NH2CH2CH2OH) were conducted to establish electrochemical reductive amination as a general route for the reductive amination of furfural-based biomass intermediates.

 Please wait while we load your content...
Please wait while we load your content...