Tin-free visible light photoredox catalysed cyclisation of enamides as a mild procedure for the synthesis of γ-lactams†
Abstract
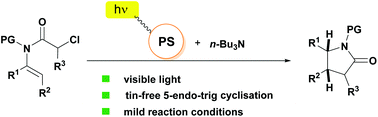
The first visible light mediated tin-free cyclisation of α-chloroenamides leading to the synthesis of substituted γ-lactams with excellent stereoselectivity is reported. The protocol employs the single-electron reduction of activated C–Cl bonds, which are typically inert towards reduction.

 Please wait while we load your content...
Please wait while we load your content...