Cooperative catalyst system for the synthesis of oleochemical cyclic carbonates from CO2 and renewables†
Abstract
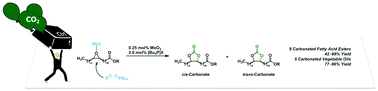
Phosphonium salts and various (transition-) metals were studied as catalysts in the synthesis of carbonated oleochemicals from the corresponding epoxides and carbon dioxide. In combination with tetra-n-butylphosphonium bromide molybdenum compounds were identified as highly active co-catalysts for the formation of cyclic carbonates. The co-catalyst accelerates the conversion of the epoxidized fatty acid ester considerably. The chemo- as well as the stereoselectivity of the carbonated oleochemicals can be controlled by the choice of the catalyst and the reaction conditions. Under optimized reaction conditions this new catalyst system allows the conversion of both mono- and polyepoxidized oleo compounds into the corresponding carbonates in good to excellent yields up to >99% under comparatively mild reaction conditions. This procedure has been applied to the synthesis of a potential renewable plasticizer and works well even at larger scale (200 g).

 Please wait while we load your content...
Please wait while we load your content...