Nanopalladium-catalyzed conjugate reduction of Michael acceptors – application in flow†
Abstract
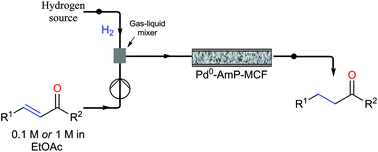
A continuous-flow approach towards the selective nanopalladium-catalyzed hydrogenation of the olefinic bond in various Michael acceptors, which could lead to a greener and more sustainable process, has been developed. The nanopalladium is supported on aminofunctionalized mesocellular foam. Both aromatic and aliphatic substrates, covering a variation of functional groups such as acids, aldehydes, esters, ketones, and nitriles were selectively hydrogenated in high to excellent yields using two different flow-devices (H-Cube® and Vapourtec). The catalyst was able to hydrogenate cinnamaldehyde continuously for 24 h (in total hydrogenating 19 g cinnanmaldehyde using 70 mg of catalyst in the H-cube®) without showing any significant decrease in activity or selectivity. Furthermore, the metal leaching of the catalyst was found to be very low (ppb amounts) in the two flow devices.


 Please wait while we load your content...
Please wait while we load your content...