An efficient sewage sludge-derived bi-functional electrocatalyst for oxygen reduction and evolution reaction†
Abstract
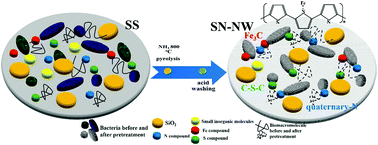
An efficient, low cost, and stable bi-functional electrocatalyst consisting of N, Fe, and S multi-doped nanoporous carbon was produced by a green and facile one-step pyrolysis of sewage sludge under NH3 conditions. The as-synthesized multi-doped electrocatalyst shows excellent electrocatalytic activities for oxygen reduction reaction (ORR) in both alkaline and acid media, which compares favorably with the commercial 20% Pt/C catalyst. The electrocatalyst also exhibits superior oxygen evolution reaction (OER) performance with favorable stability. The hierarchically porous structure, favorable surface area, and unique N, Fe, and S multi-doped carbon framework stem from the particular composition of sewage sludge and the NH3 atmosphere is responsible for the excellent catalytic efficiency of the as-synthesized electrocatalyst. This development offers a straightforward environment-compatible route for synthesizing attractive bi-functional electrocatalyst materials as promising candidates for fuel cells directly from a type of pollutant.

 Please wait while we load your content...
Please wait while we load your content...