Two angiotensin-converting enzyme-inhibitory peptides from almond protein and the protective action on vascular endothelial function
Abstract
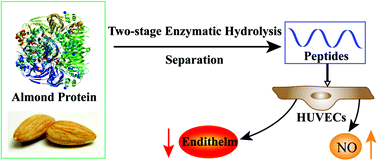
This study aimed to discover and prepare novel angiotensin converting enzyme (ACE) inhibitory peptides from almond protein and further evaluate the effect on endothelial function of human umbilical vascular endothelial cells (HUVECs). Almond protein was hydrolyzed using a two-stage alcalase–protamex hydrolysis process, and the hydrolysates were subjected to a series of separations, ultrafiltration, gel filtration chromatography, and reversed-phased preparative chromatography, to obtain the active peptides. Seven ACE inhibitory fractions with the molecular weight below 1.5 kDa were isolated and prepared, and two purified ACE inhibitory peptides with the IC50 values of 67.52 ± 0.05 and 43.18 ± 0.07 μg mL−1, were identified as Met-His-Thr-Asp-Asp and Gln-His-Thr-Asp-Asp, respectively. Then the effect of two ACE inhibitory peptides on the endothelial function of HUVECs was evaluated. Results showed that the two potent ACE inhibitory peptides significantly regulated the release of nitric oxide and endothelin in HUVECs. These results suggest that almond peptides have potential as an antihypertensive nutraceuticals or a functional food ingredient.

 Please wait while we load your content...
Please wait while we load your content...