Emerging investigators series: pyrolysis removes common microconstituents triclocarban, triclosan, and nonylphenol from biosolids†
Abstract
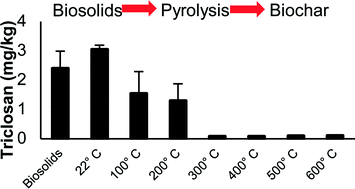
Reusing biosolids is vital for the sustainability of wastewater management. Pyrolysis is an anoxic thermal degradation process that can be used to convert biosolids into energy rich py-gas and py-oil, and a beneficial soil amendment, biochar. Batch biosolids pyrolysis (60 minutes) revealed that triclocarban and triclosan were removed (to below quantification limit) at 200 °C and 300 °C, respectively. Substantial removal (>90%) of nonylphenol was achieved at 300 °C as well, but 600 °C was required to remove nonylphenol to below the quantification limit. At 500 °C, the pyrolysis reaction time to remove >90% of microconstituents was less than 5 minutes. Fate studies revealed that microconstituents were both volatilized and thermochemically transformed during pyrolysis; microconstituents with higher vapor pressures were more likely to volatilize and leave the pyrolysis reactor before being transformed than compounds with lower vapor pressures. Reductive dehalogenation products of triclocarban and suspected dehalogenation products of triclosan were identified in py-gas. Application of biosolids-derived biochar to soil in place of biosolids has potential to minimize organic microconstituents discharged to the environment provided appropriate management of py-gas and py-oil.
- This article is part of the themed collections: Emerging Investigator Series and Environmental Science: Water Research & Technology 2016 Most Downloaded Articles

 Please wait while we load your content...
Please wait while we load your content...