Protein corona-induced modification of silver nanoparticle aggregation in simulated gastric fluid†
Abstract
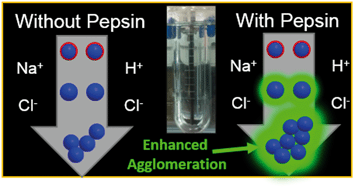
Due to their widespread incorporation into a range of biomedical and consumer products, the ingestion of silver nanoparticles (AgNPs) is of considerable concern to human health. However, the extent to which AgNPs will be modified within the gastric compartment of the gastrointestinal tract is still poorly understood. Studies have yet to fully evaluate the extent of physicochemical changes to AgNPs in the presence of biological macromolecules, such as pepsin, the most abundant protein in the stomach, or the influence of AgNPs on protein structure and activity. Herein, AgNPs of two different sizes and surface coatings (20 and 110 nm, citrate or polyvinylpyrrolidone) were added to simulated gastric fluid (SGF) with or without porcine pepsin at three pHs (2.0, 3.5, and 5.0), representing a range of values between preprandial (fasted) and postprandial (fed) conditions. Rapid increases in diameter were observed for all AgNPs, with a greater increase in diameter in the presence of pepsin, indicating that pepsin facilitated aggregation of AgNPs. AgNPs interactions with pepsin only minimally reduced the protein's proteolytic functioning capability, with the greatest inhibitory effect caused by smaller (20 nm) particles of both coatings. No changes in pepsin secondary structural elements were observed for the different AgNPs, even at high particle concentrations. This research highlights the size-dependent kinetics of nanoparticle aggregation or dissolution from interaction with biological elements such as proteins in the gastrointestinal tract. Further, these results demonstrate that, in addition to mass, knowing the chemical form and aggregation state of nanoparticles is critical when evaluating toxicological effects from nanoparticle exposure in the body.


 Please wait while we load your content...
Please wait while we load your content...