Phospholipophilicity of CxHyN+ amines: chromatographic descriptors and molecular simulations for understanding partitioning into membranes†
Abstract
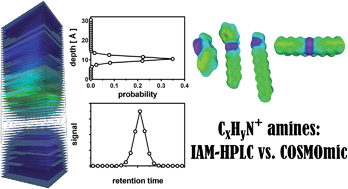
Using immobilized artificial membrane high-performance liquid chromatography (IAM-HPLC) the sorption affinity of 70 charged amine structures to phospholipids was determined. The amines contained only 1 charged moiety and no other polar groups, the rest of the molecule being aliphatic and/or aromatic hydrocarbon groups. We systematically evaluated the influence of the amine type (1°, 2°, 3° amines and quaternary ammonium), alkyl chain branching, phenyl ring positioning, charge positioning (terminal vs. central in the molecule) on the phospholipid-water partitioning coefficient (KPLIPW). These experimental results were compared with quantum-chemistry based three-dimensional (3D) molecular simulations of the partitioning of charged amines, including the most likely solute conformers, using a hydrated phospholipid bilayer in the COSMOmic module of COSMOtherm software. Both IAM-HPLC retention data and the simulations suggest that the molecular orientation of charged amines at the location in the bilayer with the lowest calculated Gibbs free energy exerts a strong influence over the partitioning within the membrane. The most favourable position of charged amines coincides with the region where the phosphate anions in the phospholipid bilayer are most abundant. Hydrocarbon units oriented in this layer are located more towards the aqueous phase and contribute less to the overall membrane affinity than hydrocarbon units extending into the more hydrophobic core of the bilayer. COSMOmic simulations explain most of the trends between the structural differences observed in IAM-HPLC based KPLIPW. For this set of cationic structures, the mean absolute difference between COSMOmic simulations and IAM-HPLC data, accounting only for amine type corrective increments, is 0.31 log units.
- This article is part of the themed collection: Emerging Investigators 2016

 Please wait while we load your content...
Please wait while we load your content...