Thermal degradation of CH3NH3PbI3 perovskite into NH3 and CH3I gases observed by coupled thermogravimetry–mass spectrometry analysis†
Abstract
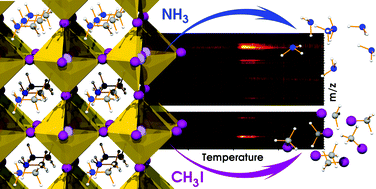
Thermal gravimetric and differential thermal analysis (TG-DTA) coupled with quadrupole mass spectrometry (MS) and first principles calculations were employed to elucidate the chemical nature of released gases during the thermal decomposition of CH3NH3PbI3. In contrast to the common wisdom that CH3NH3PbI3 is decomposed into CH3NH2 and HI, the major gases were methyliodide (CH3I) and ammonia (NH3). We anticipate that our findings will provide new insights into further formulations of the perovskite active material and device design that can prevent methylammonium decomposition and thus increase the long-term stability of perovskite-based optoelectronic devices.


 Please wait while we load your content...
Please wait while we load your content...