Highly water-soluble three-redox state organic dyes as bifunctional analytes†
Abstract
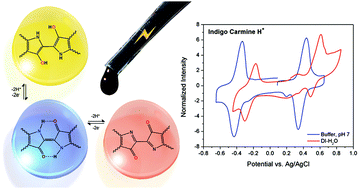
Dyes such as indigo and anthraquinone derivatives that find extensive use in industrial colorant applications contain electron density donor (–NH– or –OH) and acceptor (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups linked by conjugated bonds in their molecular structure, which are responsible for their deep shades. Departing from this stabilized redox state (“push–pull”) requires substantial energy and we found that with a proper choice of aqueous electrolyte these dyes are able to both reversibly oxidize and reduce, developing two-sets of fast two proton–electron transfers separated by ∼1 V. These large flat molecules are poorly soluble due to extensive “π” stacking, and the classical strategy of appending a sulphonate (–SO3Na) group appeared insufficient to reach ≈1 M. Herein, we have further boosted the solubility of these dyes in water up to 1.6 M by replacing Na+ with an organic cation such as tetrakis(hydroxyethyl)-ammonium [N(CH2CH2OH)4]+ or tetrakis(hydroxymethyl)-phosphonium [P(CH2OH)4]+ that are non-chelatable and very hygroscopic while maintaining a reversible charge transfer and a large electrochemical potential window in dilute protic acids (0.1 M HClO4), or in neutral pH buffered aqueous solutions (Britton–Robinson buffer). The corresponding pure salts melt even near ambient temperature. Our findings imply that the same electroactive material could be used as a catholyte and an anolyte in a 1 V symmetrical redox flow cell at neutral or close to neutral pH, with all its advantages in terms of cell corrosion and membrane requirements.
O) groups linked by conjugated bonds in their molecular structure, which are responsible for their deep shades. Departing from this stabilized redox state (“push–pull”) requires substantial energy and we found that with a proper choice of aqueous electrolyte these dyes are able to both reversibly oxidize and reduce, developing two-sets of fast two proton–electron transfers separated by ∼1 V. These large flat molecules are poorly soluble due to extensive “π” stacking, and the classical strategy of appending a sulphonate (–SO3Na) group appeared insufficient to reach ≈1 M. Herein, we have further boosted the solubility of these dyes in water up to 1.6 M by replacing Na+ with an organic cation such as tetrakis(hydroxyethyl)-ammonium [N(CH2CH2OH)4]+ or tetrakis(hydroxymethyl)-phosphonium [P(CH2OH)4]+ that are non-chelatable and very hygroscopic while maintaining a reversible charge transfer and a large electrochemical potential window in dilute protic acids (0.1 M HClO4), or in neutral pH buffered aqueous solutions (Britton–Robinson buffer). The corresponding pure salts melt even near ambient temperature. Our findings imply that the same electroactive material could be used as a catholyte and an anolyte in a 1 V symmetrical redox flow cell at neutral or close to neutral pH, with all its advantages in terms of cell corrosion and membrane requirements.

 Please wait while we load your content...
Please wait while we load your content...