Tin/vanadium redox electrolyte for battery-like energy storage capacity combined with supercapacitor-like power handling†
Abstract
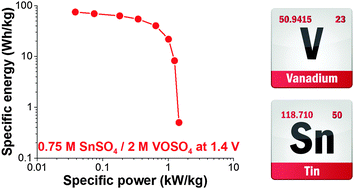
We introduce a high performance hybrid electrochemical energy storage system based on an aqueous electrolyte containing tin sulfate (SnSO4) and vanadyl sulfate (VOSO4) with nanoporous activated carbon. The energy storage mechanism of this system benefits from the unique synergy of concurrent electric double-layer formation, reversible tin redox reactions, and three-step redox reactions of vanadium. The hybrid system showed excellent electrochemical properties such as a promising energy capacity (ca. 75 W h kg−1, 30 W h L−1) and a maximum power of up to 1.5 kW kg−1 (600 W L−1, 250 W m−2), exhibiting capacitor-like galvanostatic cycling stability and a low level of self-discharging rate.

 Please wait while we load your content...
Please wait while we load your content...