Using chiral ionic liquid additives to enhance asymmetric induction in a Diels–Alder reaction†
Abstract
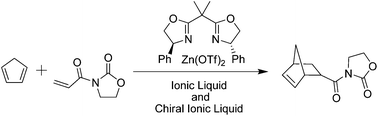
A bis-oxazoline ligand has been complexed using Cu(II) and Zn(II) trifluoromethanesulfonate and a range of chiral ionic liquid (CIL) additives based on natural products were used as a co-catalyst for a Diels–Alder reaction. The catalytic performance of these systems was compared for the asymmetric Diels–Alder reaction between N-acryloyloxazolidinone and cyclopentadiene with and without the presence of a CIL additive. In the absence of the CIL, both catalysts resulted in low enantioselectivities in conventional solvents and ionic liquids. However, whilst only a minor effect of the CIL was observed for the Cu based catalyst, in the case of the Zn based catalyst, significant enhancements in endo enantioselectivity of up to 50% were found on the addition of a CIL.


 Please wait while we load your content...
Please wait while we load your content...