Diverse structures of metal–organic frameworks via a side chain adjustment: interpenetration and gas adsorption†
Abstract
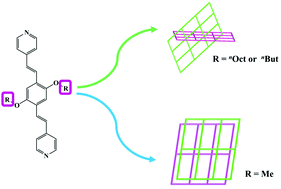
Three new coordination polymers have been synthesized based on three linear pyridine ligands with different side chains and one flexible V-shaped dicarboxylate co-ligand: {[Co(L1)0.5(sdb)]}n (1), {[Co(L2)0.5(sdb)]·1.5H2O}n (2) and {[Co(L3)0.5(sdb)]·2DMF}n (3) (L1 = E,E-2,5-dioctyloxy-1,4-bis-[2-pyridin-vinyl]-benzene; L2 = E,E-2,5-dibutoxy-1,4-bis-[2-pyridin-vinyl]-benzene; L3 = E,E-2,5-dimethoxy-1,4-bis-[2-pyridin-vinyl]-benzene; H2sdb = 4,4′-sulfonyldibenzoic acid). Complexes 1–3 are 4-connected sql nets with a point symbol of {44·62}. The interpenetration forms and porosity of frameworks have been well controlled by side chain prolongation. A larger steric hindrance for the pendant –OnOct and –OnBut groups leads to a larger repulsive force between the layers and effective construction of inclined polycatenations. A smaller steric hindrance for the pendant –Me group leads to the formation of a parallel stacked network. Therefore, compounds 1 and 2 feature 3D structures with inclined polycatenation (2D + 2D → 3D); compound 3 exhibits a non-interpenetrated 2D + 2D → 2D parallel stacked network. These complexes have been characterized by single crystal X-ray diffraction, infrared spectroscopy, thermogravimetry, elemental analysis, and powder X-ray diffraction measurements. In addition, the gas adsorption properties of the compounds have also been explored.


 Please wait while we load your content...
Please wait while we load your content...