Competing reaction pathways of 3,3,3-trifluoropropene at rhodium hydrido, silyl and germyl complexes: C–F bond activation versus hydrogermylation†
Abstract
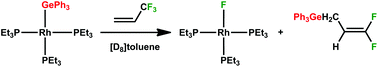
The reaction of the silyl complex [Rh{Si(OEt)3}(PEt3)3] (1) with 3,3,3-trifluoropropene afforded the rhodium complex [Rh(CH2CHCF3){Si(OEt)3}(PEt3)2] (2) which features a bonded fluorinated olefin. In contrast the rhodium hydrido complex [Rh(H)(PEt3)3] (3) yielded on treatment with 3,3,3-trifluoropropene in the presence of a base the fluorido complex [Rh(F)(PEt3)3] (4) together with 1,1-difluoro-1-propene by C–F bond activation. At low temperature the intermediate fac-[Rh(H)(CH2CHCF3)(PEt3)3] (5) was detected by NMR spectroscopy. The germyl complex [Rh(GePh3)(PEt3)3] (6) reacted also with 3,3,3-trifluoropropene by C–F bond activation affording again the fluorido complex [Rh(F)(PEt3)3] (4) as well as the (3,3-difluoroallyl)triphenylgermane 7. The catalytic hydrogermylation of 3,3,3-trifluoropropene in the presence of various germanium hydrides under mild conditions was developed by employing complex 6 as a catalyst. The molecular structures of both germane derivatives (3,3-difluoroallyl)triphenylgermane 7 and 1,1,1-trifluoropropane-3-triphenylgermane 8 were determined by X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...