Solution equilibria and the X-ray structure of Cu(ii) complexation with 3-amino-N-(pyridin-2-ylmethyl)propanamide, a pseudo-mimic of human serum albumin†
Abstract
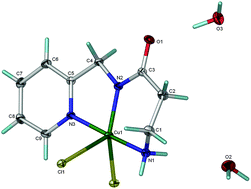
Copper complexes have anti-inflammatory activity in the treatment of inflammation associated with rheumatoid arthritis (RA). The preferred route of administration is through the skin, so the rate of dermal absorption and bioavailability of copper is important. Based on previous studies, 3-amino-N-(pyridin-2-ylmethyl)-propanamide, [H(56)NH2], was designed as a potential chelator of copper. The stability constant measurements revealed that MLH−1 is the most stable species at the physiological pH of 7.4. The X-ray crystal structure of this species was solved and copper was found in a rectangular pyramidal geometry. The ligand occupied three coordination sites while bridging chloride linked copper ions together in a chain. The ligand bound to the metal ion through the pyridyl nitrogen, the amide nitrogen and the terminal amino group. Spectroscopic studies confirmed that this structure persisted in aqueous solution. Octanol/water partition coefficients and Franz cell permeation studies showed that [H(56)NH2] is able to promote the dermal absorption of Cu(II).

 Please wait while we load your content...
Please wait while we load your content...