A Mn(iii) single ion magnet with tridentate Schiff-base ligands†
Abstract
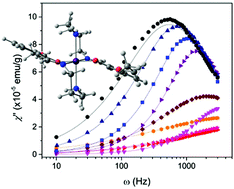
Single ion magnet behaviour is reported for a mononuclear Mn(III) ion with tridentate Schiff-base ligands which exhibits a tetragonal Jahn–Teller elongation along the Namine–Mn–Namine axis and crystallises with two crystallographically distinct Mn(III) cations (unit A and unit B). While magnetic measurements show a large and negative axial zero-field splitting (D = −4.73 cm−1), HF-EPR reveal two distinct large axial Ds (D = −4.60 cm−1 for unit A and D = −4.18 cm−1 for unit B), thus resulting in the largest D known to date for a Mn(III) single ion magnet. AC magnetic measurements at 2000 Oe allowed determination of the energy barrier for spin reversal (10.19 K) and spin reversal relaxation time (1.476 × 10−6 s) for the Mn(III) ion. Computational studies were used to characterise the electronic structure and substantiate the zero field splitting in the Mn(III) complex.


 Please wait while we load your content...
Please wait while we load your content...