Electron transfer mechanism of catalytic superoxide dismutation via Cu(ii/i) complexes: evidence of cupric–superoxo/–hydroperoxo species†
Abstract
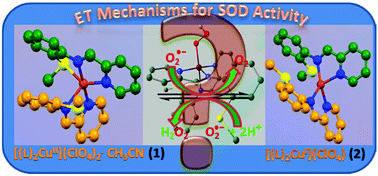
To understand the electron transfer mechanisms (outer versus inner sphere) of catalytic superoxide dismutation via a Cu(II/I) redox couple such as occur in the enzyme copper–zinc superoxide dismutase, the Cu(II/I) complexes [(L1)2Cu](ClO4)2·CH3CN, (1·CH3CN) and [(L1)2Cu](ClO4), (2) supported by a bis-N2Sthioether ligand, 2-pyridyl-N-(2′-methylthiophenyl)methyleneimine (L1) have been synthesized and structurally characterised. Both 1 and 2 display the same cyclic voltammogram (CV) featuring a quasireversible response at E1/2 = +0.33 V vs. SCE that falls in the SOD potential window of −0.04 V to +0.99 V. These complexes catalytically dismutate superoxide radicals at 298 K in aqueous medium (the IC50 for 1 is 2.15 μM). Electronic absorption spectra (233 K and 298 K), FTIR, ESI mass spectra, CV (233 K and 298 K) and DFT calculations collectively indicate formation of [(L1)2Cu(O2˙−)]+, [(L1)2Cu(O22−)] and [(L1)2Cu(OOH−)]+ species and help to elucidate the electron transfer mechanism for the SOD function of 1 and 2. Once O2˙− binds to CuII (evident at 233 K), the first step of the catalytic cycle (CuII + O2˙− → CuI + O2) does not follow but the second step (CuI + O2˙− + 2H+ → H2O2 + CuII) does follow. Therefore, the catalytic disproportionation of superoxide radicals via1 and 2 at 298 K indicates that the first and second steps of the catalytic cycle proceed through outer and inner sphere electron transfer mechanisms, respectively. Feasibility of the first step to occur in pure aprotic solvent (where 18-crown-6-ether is used to solubilise KO2) was tested and also supports the same notion of the electron transfer mechanisms as stated above.

 Please wait while we load your content...
Please wait while we load your content...