Coordination versatility of p-hydroquinone-functionalized dibenzobarrelene-based PC(sp3)P pincer ligands†
Abstract
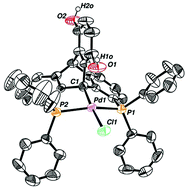
The manuscript describes the synthesis and coordination chemistry of a novel diphosphine pincer ligand based on a p-hydroquinone-functionalized dibenzobarrelene scaffold. The p-hydroquinone fragment of the ligand is oxidatively and coordinatively non-innocent and may render new reactivity to the metal center due to implied reversible redox behavior, tautomeric interconversion and metal–hydroxyl/alkoxide coordination switch of the pendant hydroxyl side-arm. Palladium, platinum and iridium complexes were prepared and characterized. Investigation of their coordination chemistry revealed that while tautomeric equilibrium exists in free ligands and in the chelate non-metalated complexes, it is essentially blocked in the corresponding C(sp3)-pincer compounds due to stabilizing hemilabile coordination of the hydroxyl group. However, its presence in close proximity to the metal center is essential for catalyzing acceptorless dehydrogenation of alcohols by the iridium complexes via the outer-sphere hydrogen transfer mechanism. Remarkably, we found a similar activity for the analogous palladium complexes, which is not characteristic of this metal. This unprecedented reactivity of palladium stresses the fact that besides the choice of an active metal, transformation-oriented design of the ligand is crucial for catalysis.
- This article is part of the themed collection: Reactions Facilitated by Ligand Design

 Please wait while we load your content...
Please wait while we load your content...