An aminopyrimidine-functionalized cage-based metal–organic framework exhibiting highly selective adsorption of C2H2 and CO2 over CH4†
Abstract
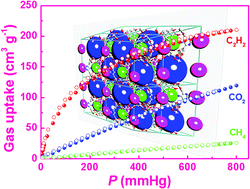
There has been considerable interest in adsorptive separation of C2H2/CH4 and CO2/CH4 gas mixtures due to its industrial significance and scientific challenge. In this work, we have designed and synthesized a bent diisophthalate ligand functionalized with aminopyrimidine groups, and constructed via a solvothermal reaction, a porous copper-based framework. Single-crystal X-ray diffraction studies show that the framework is a three-dimensional network containing three different types of polyhedral nanocages, which are stacked together to form two distinct types of one-dimensional channels along the crystallographic c axis. The compound after activation shows exceptionally high C2H2 and CO2 uptakes of 211 and 120 cm3 (STP) g−1 at 295 K and 1 atm, as well as impressive adsorption selectivities towards C2H2 and CO2 over CH4. High C2H2 and CO2 uptake capacities as well as significant adsorption selectivities of C2H2 and CO2 over CH4 imply potential applications in the adsorptive separation and purification of C2H2/CH4 and CO2/CH4 gas mixtures, which have been verified by column breakthrough experiments. Several important binding sites for C2H2 and CO2 in ZJNU-54 were revealed by quantum chemical calculations, demonstrating that the organic linkers in ZJNU-54 form unique structures that facilitate the adsorption of C2H2, while the amine groups and the Lewis basic pyrimidine-ring nitrogen sites in the organic linker improve the adsorption energies for CO2, finally leading to the increase of adsorption capacities for these two gas molecules. This work provides an efficient strategy for incorporating specific functional groups into cage-based MOFs for generating new adsorbents for highly selective gas storage and separation.

 Please wait while we load your content...
Please wait while we load your content...