Synthesis and characterisation of an N-heterocyclic carbene with spatially-defined steric impact†
Abstract
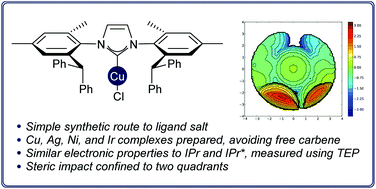
The synthesis and co-ordination chemistry of a new ‘bulky yet flexible’ N-heterocyclic carbene (“IPaul”) is reported. This carbene has spatially-defined steric impact; steric maps show that two quadrants are very bulky while the other two are quite open. The electronic properties of this carbene are very similar to those of other 1,3-diarylimidazol-2-ylidenes. Copper, silver, iridium, and nickel complexes of the new ligand have been prepared. In solution, the ligand adopts two different conformations, while X-ray crystallographic analyses of the transition metal complexes suggest that the syn-conformer is preferred in the solid state due to intermolecular interactions. The copper(I) chloride complex of this new ligand has been shown to be highly-active in the hydrosilylation of carbonyl compounds, when compared to the analogous IPr, IMes, IPr* and IPr*OMe complexes.


 Please wait while we load your content...
Please wait while we load your content...