Synthesis and electronic structure determination of uranium(vi) ligand radical complexes†
Abstract
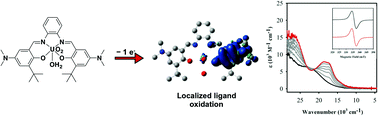
Pentagonal bipyramidal uranyl (UO22+) complexes of salen ligands, N,N′-bis(3-tert-butyl-(5R)-salicylidene)-1,2-phenylenediamine, in which R = tBu (1a), OMe (1b), and NMe2 (1c), were prepared and the electronic structure of the one-electron oxidized species [1a–c]+ were investigated in solution. The solid-state structures of 1a and 1b were solved by X-ray crystallography, and in the case of 1b an asymmetric UO22+ unit was found due to an intermolecular hydrogen bonding interaction. Electrochemical investigation of 1a–c by cyclic voltammetry showed that each complex exhibited at least one quasi-reversible redox process assigned to the oxidation of the phenolate moieties to phenoxyl radicals. The trend in redox potentials matches the electron-donating ability of the para-phenolate substituents. The electron paramagnetic resonance spectra of cations [1a–c]+ exhibited gav values of 1.997, 1.999, and 1.995, respectively, reflecting the ligand radical character of the oxidized forms, and in addition, spin–orbit coupling to the uranium centre. Chemical oxidation as monitored by ultraviolet-visible-near-infrared (UV-vis-NIR) spectroscopy afforded the one-electron oxidized species. Weak low energy intra-ligand charge transfer (CT) transitions were observed for [1a–c]+ indicating localization of the ligand radical to form a phenolate/phenoxyl radical species. Further analysis using density functional theory (DFT) calculations predicted a localized phenoxyl radical for [1a–c]+ with a small but significant contribution of the phenylenediamine unit to the spin density. Time-dependent DFT (TD-DFT) calculations provided further insight into the nature of the low energy transitions, predicting both phenolate to phenoxyl intervalence charge transfer (IVCT) and phenylenediamine to phenoxyl CT character. Overall, [1a–c]+ are determined to be relatively localized ligand radical complexes, in which localization is enhanced as the electron donating ability of the para-phenolate substituents is increased (NMe2 > OMe > tBu).

 Please wait while we load your content...
Please wait while we load your content...