Chelating N-heterocyclic carbene–carboranes offer flexible ligand coordination to IrIII, RhIII and RuII: effect of ligand cyclometallation in catalytic transfer hydrogenation†
Abstract
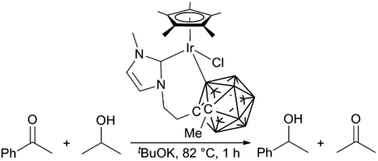
Imidazolium salts linked by an ethyl tether to closo-dicarbadodecaboranes were reacted with [IrCp*Cl2]2, [RhCp*Cl2]2 or [Ru(p-cymene)Cl2]2 in the presence of Ag2O to prepare complexes of the type [MCp*(NHC)Cl2] (M = Ir, Rh; NHC = N-heterocyclic carbene) or [Ru(p-cymene)(NHC)Cl2]. When the NHC contained an N-tBu substituent, C–H activation of the tBu and subsequent alkyl coordination was observed at Ir. Coordination of the closo-dicarbadodecaborane moiety to Ir was possible to give 7-membered metallacycles, coordinated through the carbenic carbon of the NHC and either a carbon atom or a boron atom of the carborane. Examination of the Ir complexes in the transfer hydrogenation of acetophenone to 1-phenylethanol reveals that cyclometallation of the carborane moiety is important for catalytic efficacy, indicating a bifunctional mechanism and involvement of the dicarbadodecaborane anion.
- This article is part of the themed collection: Reactions Facilitated by Ligand Design

 Please wait while we load your content...
Please wait while we load your content...