Unveiling the adsorption mechanism of zeolitic imidazolate framework-8 with high efficiency for removal of copper ions from aqueous solutions†
Abstract
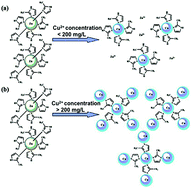
Among the heavy metal ions, copper(II) can cause eye and liver damage at high uptake. The existence of copper ions (Cu2+) even with an ultralow concentration of less than 0.1 μg g−1 can be toxic to living organisms. Thus, it is highly desirable to develop efficient adsorbents to remove Cu2+ from aqueous solutions. In this work, without any surface functionalization or pretreatment, a water-stable zeolitic imidazolate framework (ZIF-8) synthesized at room temperature is directly used as a highly efficient adsorbent for removal of copper ions from aqueous solutions. To experimentally unveil the adsorption mechanism of Cu2+ by using ZIF-8, we explore various effects from a series of important factors, such as pH value, contact time, temperature and initial Cu2+ concentration. As a result, ZIF-8 nanocrystals demonstrate an unexpected high adsorption capacity of Cu2+ and high removal efficiency for both high and low concentrations of Cu2+ from water. Moreover, ZIF-8 nanocrystals possess fast kinetics for removing Cu2+ with the adsorption time of less than 30 min. In addition, the pH of the solution ranging from 3 to 6 shows little effect on the adsorption of Cu2+ by ZIF-8. The adsorption mechanism is proposed for the first time and systematically verified by various characterization techniques, such as TEM, FTIR, XPS, XRD and SEM.

 Please wait while we load your content...
Please wait while we load your content...