Electrophilic activation of alkynes for enyne cycloisomerization reactions with in situ generated early/late heterobimetallic Pt–Ti catalysts†
Abstract
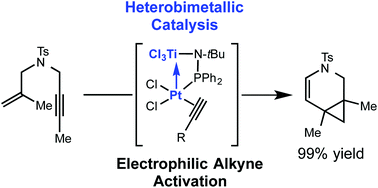
In situ formation of heterobimetallic Pt–Ti catalysts enables rapid room temperature catalysis in enyne cycloisomerization reactions. The Lewis acidic titanium atom in the ligand framework is shown to be essential for fast catalysis. A range of enyne substrates are efficiently cyclized to carbocycles and heterocycles in high yield.
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...