Lewis acid–base adducts of group 13 elements: synthesis, structure and reactivity toward benzaldehyde†
Abstract
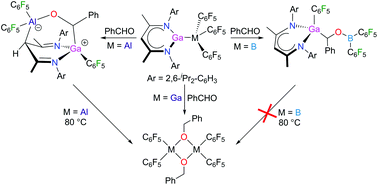
Lewis acid–base adducts [LGa-M(C6F5)3] (M = B 1, Al 2, Ga 3) were prepared by the reaction of gallanediyl LGa {L = HC[C(Me)N(2,6-i-Pr2C6H3)]2} with the Lewis acids M(C6F5)3 (M = B, Al, Ga). Benzaldehyde reacts with [LGa-M(C6F5)3] (M = B 1, Al 2) at room temperature with the insertion and formation of [LGa(C6F5){CH(Ph)(OB(C6F5)2)}] (4) and the zwitterionic species [LGa(C6F5){CH(Ph)(OAl(C6F5)2)}] (5), respectively, which was found to decompose at 80 °C with the formation of {(C6F5)2Al(OCH2Ph)}2 (6). Any attempts to isolate the insertion complex of [LGa-Ga(C6F5)3] with benzaldehyde failed and only {(C6F5)2Ga(OCH2Ph)}2 (7) was isolated at elevated temperatures. 2–5 and 7 were structurally characterized by heteronuclear NMR spectroscopy and single crystal X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...