Activation of heteroallenes by coordinatively unsaturated nickel(ii) alkyl complexes supported by the hydrotris(3-phenyl-5-methyl)pyrazolyl borate (TpPh,Me) ligand†
Abstract
Activation of sulfur containing heteroallenes by nickel(II) alkyl complexes supported by the bulky hydrotris(3-phenyl-5-methylpyrazolyl)borate (TpPh,Me) ligand is described. Exposure of TpPh,MeNiCH2Ph (1a) and TpPh,MeNiCH2Si(CH3)3 (1b) to CS2 resulted in formation of the insertion products TpPh,MeNi(η2-CS2)CH2Ph (2a) and TpPh,MeNi(η2-CS2)CH2Si(CH3)3 (2b) in moderate yields. Reaction of 1a and MeNCS produced two species in a 1 : 1 ratio, identified as TpPh,MeNi(η2-MeNC)CH2Ph (3) and TpPh,MeNi(η2-MeNCS)SCH2Ph (4). Isolation of the unexpected insertion product (3) prompted an investigation into the activity of 1a–b in the presence of isocyanides (i.e.tBuNC), which resulted in isolation of TpPh,MeNi(η2-tBuNC)CH2Ph (5a) and TpPh,MeNi(η2-tBuNC)CH2Si(CH3)3 (5b). Similarly, reaction of 1a with OCS led to the isolation of a rare example of a Ni(I) carbonyl species TpPh,MeNiCO (6). Alternatively, complex 6 was also formed by exposure of 1a–b to an atmosphere of CO. Isolation of the intermediate species (TpPh,MeNi(η2-CO)CH2TMS (7b) and TpPh,MeNi(CO)(C(O)R, (8a–b) with R = Ph, TMS)) shed light on the formation of such species.
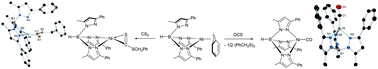
- This article is part of the themed collection: Small Molecule Activation

 Please wait while we load your content...
Please wait while we load your content...