RuII, IrIII and OsII mesoionic carbene complexes: efficient catalysts for transfer hydrogenation of selected functionalities†‡
Abstract
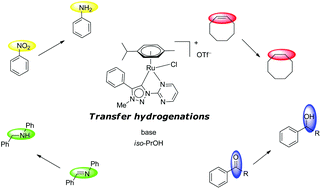
Pyridine-appended triazolylidene donors have been recently used as ligands in various homogeneous catalytic processes. We present here a new pyrimidine substituted triazolium salt which was prepared and used in the coordination to RuII and OsII. The new triazolium salt and the obtained complexes were characterized by multinuclear NMR spectroscopy. The molecular composition of the mentioned compounds was confirmed by positive ion electrospray ionization (ESI+) mass spectra. The new pyrimidyl containing complexes, as well as the related pyridyl-triazolylidene containing complexes, were applied in transfer hydrogenation reactions of carbonyls, alkenes, imines and nitroarenes. The pyrimidyl containing complexes reveal an over-all better activity in comparison to their pyridine bearing analogues. The studies of electronic effects of the ligands, as well as mechanistic insights for the reduction of nitrobenzene with three selected precatalysts are presented.
- This article is part of the themed collection: Reactions Facilitated by Ligand Design

 Please wait while we load your content...
Please wait while we load your content...