Tris(pentafluorophenyl)borane as an efficient catalyst in the guanylation reaction of amines†
Abstract
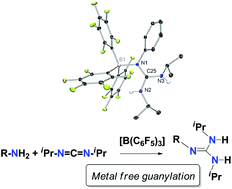
Tris(pentafluorophenyl)borane, [B(C6F5)3], has been used as an efficient catalyst in the guanylation reaction of amines with carbodiimide under mild conditions. A combined approach involving NMR spectroscopy and DFT calculations was employed to gain a better insight into the mechanistic features of this process. The results allowed us to propose a new Lewis acid-assisted Brønsted acidic pathway for the guanylation reaction. The process starts with the interaction of tris(pentafluorphenyl)borane and the amine to form the corresponding adduct, [(C6F5)3B-NRH2] 1, followed by a straightforward proton transfer to one of the nitrogen atoms of the carbodiimide, iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr, to produce, in two consequent steps, a guanidine–borane adduct, [(C6F5)3B-NRC(NiPrH)2] 2. The rupture of this adduct liberates the guanidine product RNC(NiPrH)23 and interaction with additional amine restarts the catalytic cycle. DFT studies have been carried out in order to study the thermodynamic characteristics of the proposed pathway. Significant borane adducts with amines and guanidines have been isolated and characterized by multinuclear NMR in order to study the N–B interaction and to propose the existence of possible Frustrated Lewis Pairs. Additionally, the molecular structures of significant components of the catalytic cycle, namely 4-tert-butylaniline-[B(C6F5)3] adduct 1b and both free and [B(C6F5)3]-bonded 1-(phenyl)-2,3-diisopropylguanidine, 2a and 3a respectively, have been established by X-ray diffraction.
NiPr, to produce, in two consequent steps, a guanidine–borane adduct, [(C6F5)3B-NRC(NiPrH)2] 2. The rupture of this adduct liberates the guanidine product RNC(NiPrH)23 and interaction with additional amine restarts the catalytic cycle. DFT studies have been carried out in order to study the thermodynamic characteristics of the proposed pathway. Significant borane adducts with amines and guanidines have been isolated and characterized by multinuclear NMR in order to study the N–B interaction and to propose the existence of possible Frustrated Lewis Pairs. Additionally, the molecular structures of significant components of the catalytic cycle, namely 4-tert-butylaniline-[B(C6F5)3] adduct 1b and both free and [B(C6F5)3]-bonded 1-(phenyl)-2,3-diisopropylguanidine, 2a and 3a respectively, have been established by X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...