Synergistic oxygen atom transfer by ruthenium complexes with non-redox metal ions†
Abstract
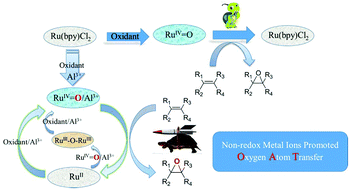
Non-redox metal ions can affect the reactivity of active redox metal ions in versatile biological and heterogeneous oxidation processes; however, the intrinsic roles of these non-redox ions still remain elusive. This work demonstrates the first example of the use of non-redox metal ions as Lewis acids to sharply improve the catalytic oxygen atom transfer efficiency of a ruthenium complex bearing the classic 2,2′-bipyridine ligand. In the absence of Lewis acid, the oxidation of ruthenium(II) complex by PhI(OAc)2 generates the Ru(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species, which is very sluggish for olefin epoxidation. When Ru(bpy)2Cl2 was tested as a catalyst alone, only 21.2% of cyclooctene was converted, and the yield of 1,2-epoxycyclooctane was only 6.7%. As evidenced by electronic absorption spectra and EPR studies, both the oxidation of Ru(II) by PhI(OAc)2 and the reduction of Ru(IV)
O species, which is very sluggish for olefin epoxidation. When Ru(bpy)2Cl2 was tested as a catalyst alone, only 21.2% of cyclooctene was converted, and the yield of 1,2-epoxycyclooctane was only 6.7%. As evidenced by electronic absorption spectra and EPR studies, both the oxidation of Ru(II) by PhI(OAc)2 and the reduction of Ru(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O by olefin are kinetically slow. However, adding non-redox metal ions such as Al(III) can sharply improve the oxygen transfer efficiency of the catalyst to 100% conversion with 89.9% yield of epoxide under identical conditions. Through various spectroscopic characterizations, an adduct of Ru(IV)
O by olefin are kinetically slow. However, adding non-redox metal ions such as Al(III) can sharply improve the oxygen transfer efficiency of the catalyst to 100% conversion with 89.9% yield of epoxide under identical conditions. Through various spectroscopic characterizations, an adduct of Ru(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O with Al(III), Ru(IV)
O with Al(III), Ru(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/Al(III), was proposed to serve as the active species for epoxidation, which in turn generated a Ru(III)–O–Ru(III) dimer as the reduced form. In particular, both the oxygen transfer from Ru(IV)
O/Al(III), was proposed to serve as the active species for epoxidation, which in turn generated a Ru(III)–O–Ru(III) dimer as the reduced form. In particular, both the oxygen transfer from Ru(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/Al(III) to olefin and the oxidation of Ru(III)–O–Ru(III) back to the active Ru(IV)
O/Al(III) to olefin and the oxidation of Ru(III)–O–Ru(III) back to the active Ru(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/Al(III) species in the catalytic cycle can be remarkably accelerated by adding a non-redox metal, such as Al(III). These results have important implications for the role played by non-redox metal ions in catalytic oxidation at redox metal centers as well as for the understanding of the redox mechanism of ruthenium catalysts in the oxygen atom transfer reaction.
O/Al(III) species in the catalytic cycle can be remarkably accelerated by adding a non-redox metal, such as Al(III). These results have important implications for the role played by non-redox metal ions in catalytic oxidation at redox metal centers as well as for the understanding of the redox mechanism of ruthenium catalysts in the oxygen atom transfer reaction.

 Please wait while we load your content...
Please wait while we load your content...