Exposing elusive cationic magnesium–chloro aggregates in aluminate complexes through donor control†
Abstract
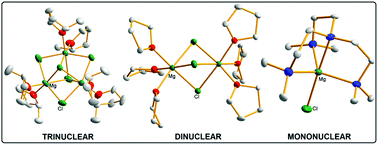
The cationic magnesium moiety of magnesium organohaloaluminate complexes, relevant to rechargeable Mg battery electrolytes, typically takes the thermodynamically favourable dinuclear [Mg2Cl3]+ form in the solid-state. We now report that judicious choice of Lewis donor allows the deliberate synthesis and isolation of the hitherto only postulated mononuclear [MgCl]+ and trinuclear [Mg3Cl5]+ modifications, forming a comparable series with a common aluminate anion [(Dipp)(Me3Si)NAlCl3]−. By pre-forming the Al–N bond prior to introduction of the Mg source, a consistently reproducible protocol is reported. Usage of the green solvent 2-methyltetrahydrofuran in place of THF in the context of Mg/Al battery electrolyte type complexes is also promoted.

 Please wait while we load your content...
Please wait while we load your content...